
This set of laboratories houses instruments used for the chemical and morphological characterization of the atmospheric particulate that is collected during studies and measurement campaigns of indoor and outdoor air quality, carried out in areas remote, rural, industrial and urban. The whole of the instrumentation allows to carry out a complete evaluation of the chemical composition of the particulate which, in turn, allows to identify and estimate the strength of its main sources.
INSTRUMENTS
Balance room
In the laboratory there are 2 Crystal Gibertini analytical balances with a sensitivity of 0.1 milligrams, and a Sartorius ME5 automated balance, with a sensitivity of 1 microgram. All the scales are housed in conditioned chambers at constant temperature and humidity. The automated scale, equipped with a computer-controlled robotic system, is used to measure the mass of atmospheric dust collected on the filter membranes.

XRF laboratory
The laboratory houses a Xepos Spectro energy dispersion X-ray fluorescence spectrophotometer, for the determination of the elements contained in atmospheric particulate samples. The non-destructive technique is used at the beginning of the analytical process for determining the chemical composition of atmospheric dust, immediately after determining the mass collected, and allows further analyzes to be carried out on the same sample.
Description
The laboratory houses a Xepos Spectro energy dispersion X-ray fluorescence spectrophotometer, for the determination of the elements contained in atmospheric particulate samples. The non-destructive technique is used at the beginning of the analytical process for determining the chemical composition of atmospheric dust, immediately after determining the mass collected, and allows further analyzes to be carried out on the same sample.
The ED – XRF technique allows to identify and quantify the elemental composition of samples with bulk (massive sample) or particulate (particulate sample) matrix.
The sample is bombarded with a high-energy X-ray beam generated by a source (X-ray tube) inside the instrument. A portion of X-rays is absorbed by the sample, bringing the atomic species that compose it to an excited energetic state. In returning to the fundamental energy state, the excited atoms emit photons with lower energy than the incident beam, with fluorescence. Each atomic species emits characteristic energy bands, which makes it possible to identify the species present in the sample, and the intensity of the radiation emitted by each species is proportional to its concentration in the sample itself. Given the high energy and intensity of the incident X-ray beam, the fluorescence excitation / emission process affects the entire mass of the analyzed sample, allowing to determine its elemental composition in a quantitative manner.
The determination of all atomic species is carried out simultaneously, by means of an energy dispersion semiconductor detector and a multichannel analyzer. The concentration of each element is then calculated by means of a calibration curve with standards or samples of known concentration.
At the CNR-IIA Metals Laboratory there is an ED-XRF SPECTRO XEPOS (AMETEK) with 50 W / 60 kV X-ray tube and dedicated quantitative analysis software (TurboQuant II, AMETEK).

Inorganic pollutants preparation laboratory
The laboratory is equipped with a Merck MilliQ / Elix3 deionized water production system, ultrasonic baths and equipment needed to prepare the samples for subsequent instrumental analysis.
In the case of atmospheric dust collected on the filter membrane, the water-soluble chemical substances are extracted in aqueous solution by immersing the sample in the ultrasonic bath or using mechanical stirrers. The subsequent chemical analyzes are carried out on the solutions thus obtained.

Ion chromatography laboratory
In the laboratory there are two Aquion and Ics1000 Thermo Fisher ion chromatographs, equipped with an autosampler, for the analysis of ionic species (anions and cations) and a 930 Compact Ic Flex Metrohm ion chromatograph with pulsed amperometry detector, equipped with an autosampler, for 'analysis of anhydro-sugars.
After the solubilization phase, the aqueous solutions containing the atmospheric dust samples are analyzed to determine their content in traditional water-soluble species (anions, cations) and in anhydro-sugars, including levoglucosane, an excellent tracer of biomass combustion.

ICP laboratory
In the laboratory there is a mass spectrometer with inductively coupled plasma detector (ICP-MS) X Series II Thermo for the analysis of the content in micro-elements and trace elements, and an Ethos 1_Milestone microwave mineralizer for the complete dissolution of solid samples. The analyzes in ICP-MS allow to simultaneously determine a very high number of elements present in the atmosphere even at very low concentration, complementing the determinations carried out by ED-XRF.
Description
Inductively Coupled Plasma Mass Spectrometry (ICP - MS) is a fast, versatile and sensitive multi-element analysis technique that enables the determination of trace metals in complex organic and inorganic matrices.
The analytical technique uses the heat of a plasma torch to ionize the sample. The plasma is generated from a flow of Argon gas and can reach a temperature between 6.000 and 10.000 K.
The spectrometer uses a quadrupole mass analyzer. The ions are separated on the basis of their mass / charge ratio; the generated signal is proportional to the concentration. The concentration of the single element is determined by calibration with standards of known concentration. In order to compensate for the variability of the measurement caused by the “matrix effect”, it is possible to add known quantities of one or more selected elements (internal standards) to both the samples and the solutions used for calibration.
The sample preparation involves the complete solubilization of the sample through an acid mineralization process with microwaves.
The application of the ICP - MS technique to the analysis of environmental matrix samples allows the determination of all metals present, even in traces, in the samples taken both in ambient air and in industrial or indoor sites.

Thermo-optical analysis laboratory
The laboratory houses the instrumentation necessary for the analysis of elemental carbon and of the set of organic species present in atmospheric dust. It includes a high temperature muffle furnace for the pretreatment of the filter membranes used for the sampling of the organic fraction, two benchtop thermo-optical analyzers Lab OC / EC Aerosol Analyzer, Sunset Laboratory, and an analogue thermo-optical analyzer with high temporal resolution (1 hour) for field analysis (Model-4 Semi-Continuous OC-EC Field Analyzer, Sunset Laboratory).
Description
The instrument directly analyzes the airborne dust sampled on the filter membrane without the need for any pre-treatment. However, given the extreme thermal conditions to which the samples are subjected, it is necessary to use specific membranes in quartz fiber, which are thermally resistant to the operating temperatures of the instrument.
The sample is in fact subjected to two distinct high-temperature heat treatments, the first of which involves the determination of organic carbon by thermal desorption in an inert atmosphere (helium), following a thermal ramp that progressively reaches a temperature of 870 ° C. After a phase of partial cooling, the sample is again subjected to a thermal ramp in an oxidizing atmosphere (10% oxygen), up to a temperature of 890 ° C, for the determination of elemental carbon.
All organic species released from the sample during the entire thermal protocol are transported to a catalyst oven containing manganese dioxide (MnO2) and completely oxidized to carbon dioxide (CO2). Following a reduction process, the CO2 is then transformed into methane, which is quantified using a flame ionization detector (FID).
During the whole process the instrument continuously monitors the transmittance value of a laser radiation incident on the sample surface. A drawback of this heat treatment is in fact represented by the so-called charring (or blackening of the filter), due to the formation of pyrolytic carbon during the first phase of heating in an inert atmosphere, which would be erroneously quantified as elemental carbon. In this phase the charring causes the reduction of the transmittance signal, while during the following phase, in an oxidizing atmosphere, a progressive increase of the signal is recorded, which reaches its initial value at the end of the pyrolysis process. It is at this point that the system determines the pyrolyzed material, which allows the correct attribution of the OC and EC values.
The use of this instrumentation has the main advantage of determining with a single, rapid analysis, and without any pretreatment of the sample, the two carbon fractions which in the total mass balance of PM represent a large part of the total mass of the sampled particles.
The disadvantage of this technique is instead that of not being able to carry out any speciation of the organic fraction in the various subclasses of compounds, which is possible only by using expensive and demanding analytical methods and instrumentation both in the sample preparation phase and during the actual analysis.
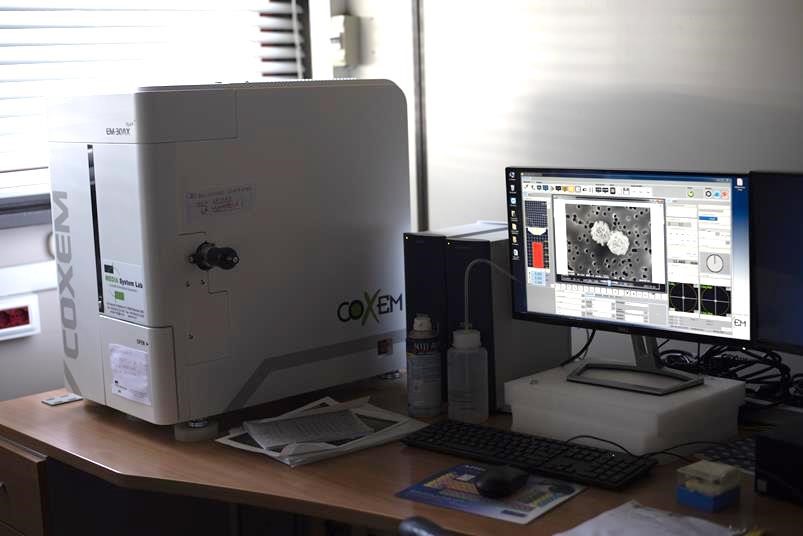
Electron microscopy laboratory
The laboratory houses a Coxem EM-30AX Plus scanning electron microscope, coupled with energy dispersion spectroscopy (SEM-EDS). The instrument allows to perform the morphological analysis of the particulate samples and to generate high resolution images of the dust collected on the sampling membrane; it also allows to perform the qualitative-quantitative chemical characterization of the single particles
Description
The SEM - EDS technique allows to obtain chemical-physical and morphological information of samples with bulk (massive sample) or particulate (particulate sample) matrix of any origin and nature, through the interaction between an electron beam of adequate energy and the matrix same as the sample.
The electron beam, generated by a source (gun), is accelerated along the electronic column up to a vacuum chamber, where the sample is inserted, on which it carries out the scan of different points or areas. During the scan, the electrons of the beam (primary) interact with the analyzed material. This interaction induces the emission from the sample of electrons at different energy, and from different depth levels of the material (mainly Auger electrons, secondary and backscattered), as well as of X-rays characteristic of the chemical elements present in its composition.
The Auger, secondary and backscattered electrons give information on, respectively, the composition of the surface layer, the morphology of the surface and the average atomic number at the point of analysis.
The characteristic X-rays describe its elemental composition. This is determined by microanalysis of the X emission in energy dispersion spectroscopy (EDS), and subsequent spectral interpretation. In the case of particle samples, the aforementioned morpho-chemical information can also be obtained from the individual particles, which allows to greatly amplify the information content of the SEM-EDS microanalysis. In environmental applications this allows, for example, to trace the sources and factors responsible for the composition of the sample. At the CNR-IIA Microscopy Laboratory there is a SEM-EDS COXEM EM-30AX Plus with thermionic emission W electronic source, combined with SDD detector for EDS (Oxford Instruments) microanalysis.
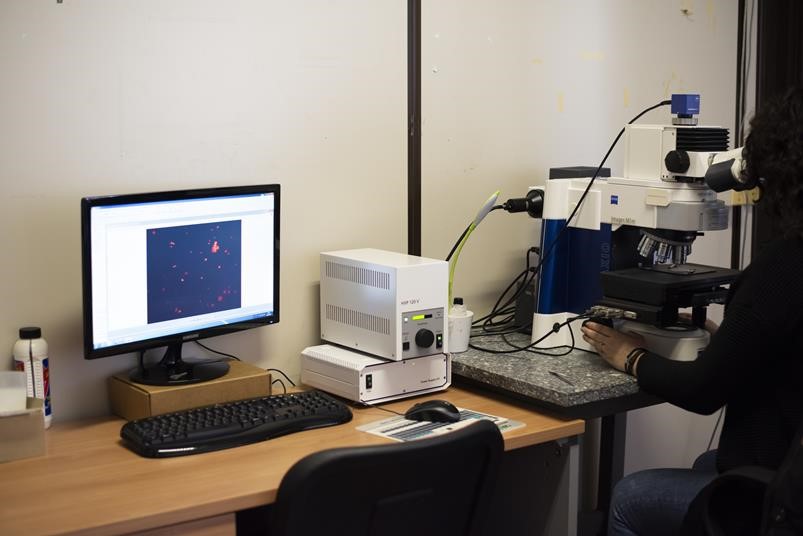
Microscopy laboratory octave
The laboratory is equipped with an Imager M1m Zeiss optical microscope with magnification from 5x to 100x and a high resolution Axiocam Zeiss camera for image acquisition. It is used to carry out the morphological characterization of the particulate and for the determination of the bioaerosol content by means of a dedicated technique which involves selective staining with propidium iodide and subsequent observation in fluorescence.
Description
Optical microscopy is an analytical technique that exploits the principle of light reflection by means of a system of lenses and mirrors, to optically magnify a solid or liquid body when it is illuminated by a source.
The optical microscope in use at the Institute is mainly intended for the morphological analysis of atmospheric particulate samples. The application of the optical microscopy technique to the study of airborne particulate material allows the discrimination and characterization of individual particles according to parameters such as size, shape, color and surface roughness.
The microscope used at IIA is also equipped with an epifluorescence system: the light source emits a beam that passes through the objective and is absorbed by the sample. The latter generates a fluorescence signal due to the emission of a light beam with a wavelength different from that absorbed. The wavelengths relating to absorption and emission are regulated by an optical filter positioned between the objective and the detector.
In environmental research, the epifluorescence microscope allows the study of specific classes of airborne particles capable of generating fluorescence if treated with a specific marker. In the case of bioaerosol, for example, the marker binds to the DNA or RNA molecules present in the particles that make up the sample, generating a fluorescence signal. The observation of the sample by means of epifluorescence microscopy and the processing of the images acquired through a specific software allow the estimation of the mass concentration of the airborne biological material.
